usp class vi compliant
Request a Quote Now. Testing was performed by Pacific BioLabs on September 16 2015 in compliance with the standards published in the USP Biocompatibility Testing standards USP.
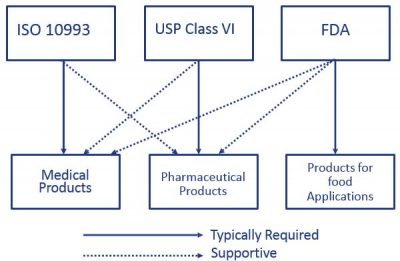
The USP defines six plastics classes from class I to class VI with class VI being the most rigorous and most frequently requested certification.

. One standard often overlooked but usually published alongside USP Class VI is FDA 21 CFR 1772600. PPE manufactures medical and pharmaceutical grade gaskets and seals including O-rings sanitary gaskets and other high performance seals from a range of 15 USP Class VI compliant elastomers-. Compounds made without animal-derived ingredients BSETSE concerns.
Usp class vi chapter 88 relates to in vivo biological reactivity tests its purpose is to determine the biological. Results are as follows. Professional Plastics General Product Range Brochure.
SIMONA PP-H USP Class VI sheet material is easy to clean and disinfect using most hospital grade cleaners and disinfectants. This material is also BPA Lead and heavy metal free phthalates safe and is REACH RoHS DMF and FDA compliant. USP Class VI compliant.
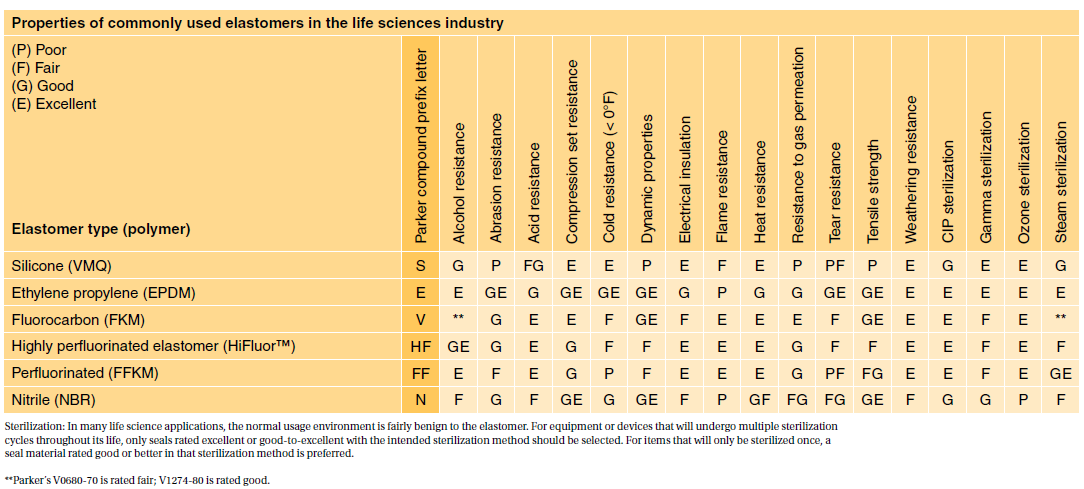
Three chapters are applicable to elastomers plastics and polymeric materials. Usp class vi compliant products newman sanitary gasket company is the only gasket oring and custom molding manufacturer to offer you technology that has allowed eleven of our exclusive elastomer compounds to receive class vi compliant certification. Specifically USP publishes test instructions for the plastics polymers and elastomers that are used in medical devices and surgical equipment.
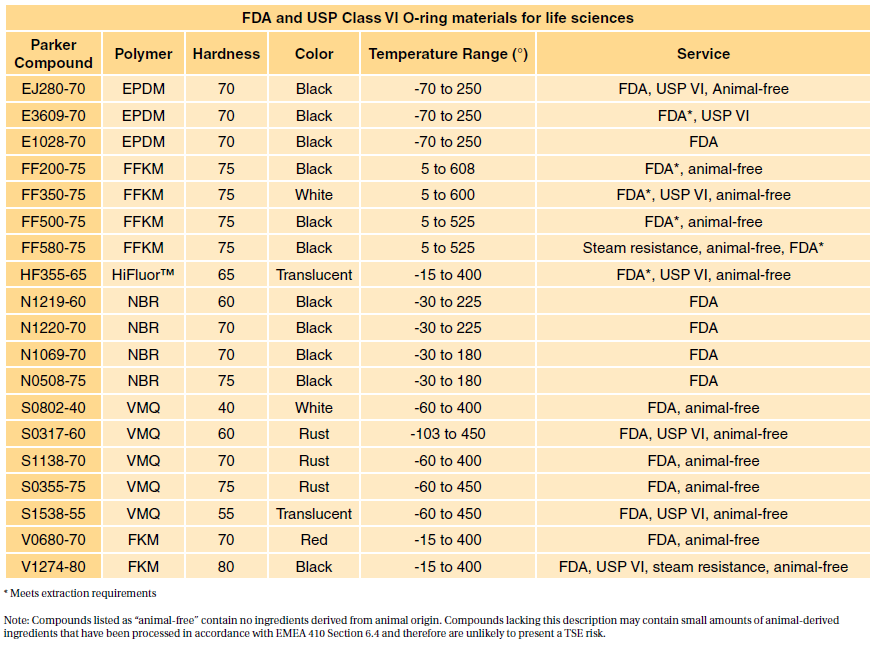
FDA Compliant and USP Class VI Compliant Elastomers. Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials. Sil 714001 USP class VI Silicone 1 70 Yes transl.
Typical applications for our FDA NSF 51 USDA materials are disposable medical. There are six classes VI being the most rigorous. Pharmacopeia USP a non-profit organization whose standards inform decision-making at the US.
Consumers implicitly rely upon the standards put into place by governing agencies to protect the publics health and well-being. Some medical silicones must meet USP Class VI FDA CFR 21 1772600 and RoHS requirements. Medical Grade Polypropylene Our USP class VI polypropylene is 100 virgin medical lab and food grade and is even autoclave sterilizable.
Pharmacopoeia Class VI judges the suitability of plastic material intended for use as containers or accessories for parenteral preparations. All these special grade products have passed this rigorous test. Biological Test for Plastics USP Class VI 121oC E553 Article meets the requirements.
Graco Company have been tested for compliance to USP Class VI 70C plastic. Many plastics manufacturers find it advantageous to have their materials classified especially if their plastic resins are a likely candidate to be used in medical devices. Tests of the provided material samples passed all requirements and have been approved for.
ADI-free certifies that the raw materials used in production of the elastomer contain no Animal Derived Ingredients ADI. Enflo products are USP Class VI FDA ROHS REACH and Conflict Materials compliant. The United States Pharmacopoeia USP 30 NF 25 2007 standard also known as Class VI is widely used to comply with stringent FDA regulations for products that come in contact with the human body.
I - VI with USP Class VI being the strictest requiring that the material exhibit very low levels of toxicity proven. When production of the elastomer contain no ADI with respect to source manufacture and treatment they cannot. Food and Drug Administration FDA.
Table 1 shows our standard programme FDA compliant com- FDA and USP class VI compliant. USP Class VI refers to a set of biocompatibility testing requirements from the US. What is ADI-Free BSE-Free TSE-Free.
Suitability under USP Class VI is typically a base requirement for medical device manufacturers. Pharmacopoeia USP Class VI outlines requirements for system toxicity and intracutaneous toxicity for these cleaner compounds. Compliance to USP Class VI is often requested by users in the biopharmaceutical and medical industries.
ISO 90012015 Certified QMS. RoHS a European Union Directive restricts the use of certain substances but manufacturers also need to know whether all the ingredients in a medical silicone are made of compliant materials. Master Bond systems are very versatile and can be used for both disposable and reusable medical devices.
Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal. The USP Class VI compounds must be made from ingredients with clear histories of biocompatibility that meet tighter requirements for leachates. When evaluating a new product many of our customers immediately jump to USP Class VI approval tests.
USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials. Sil 714002 USP class VI Silicone 1 70 Yes transl. ENFLON is a registered trademark for.
Has a full range of specialty adhesives epoxies primers for polyolefins UV curables and silicones that have been fully tested to meet USP Class VI requirements. United States Pharmacopeia USP 26 NF21 2003 Class VI. This entry was posted in Mentor Nuggets and tagged autoclavable black ultem rod black ultem sheet buy ultem rod buy ultem sheet FDA compliant peek rod peek sheet ppsu rod ppsu sheet ptfe rod ptfe sheet radel rod radel sheet sabic ultem resin shortage shop for ultem rod shop for ultem sheet UL 94 V-0 ultem 1000 rod ultem 1000.
The sample is designated as E553 Article within our test report for samples submitted July 2014. SIMONA PP-H USP Class VI sheet is ideal for applications requiring biocompatibility testing standards defined by ISO 109931. The USP outlines classes for plastic materials ie.
27 rows The US. In addition SIMONA PP-H USP Class VI sheet delivers high chemical and corrosion resistance excellent surface. Ethylene Propylene is a elastomer that demonstrates great weather aging and ozone resistance excellent wat Home current.
Time tested for USP Class VI compliance This article was manufactured using procedures typically required to produce final parts. USP Class Testing standards are determined by the United States. 7 USP Class VI materials EPDM silicone fluorocarbon and perfluoroelastomer 24 materials which are compliant to FDA 21 CFR1772600.
Specially formulated for long term sealing.

Dursan Passes Usp Class Vi Testing Why Is That Important

Usp Class Plastics Pacific Biolabs
Dursan Passes Usp Class Vi Testing Why Is That Important

What Is Usp Class Vi Testing Tbl Plastics
Material Selection Medical Injection Molding Xcentric Mold

Usp Class Vi Foster Corporation

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

1 25 1 1 4 Id Fda Usp Class Vi Platinum Silicone W Polyester Braid Food And Pharma Grade Flex Technologies Incorporated

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc
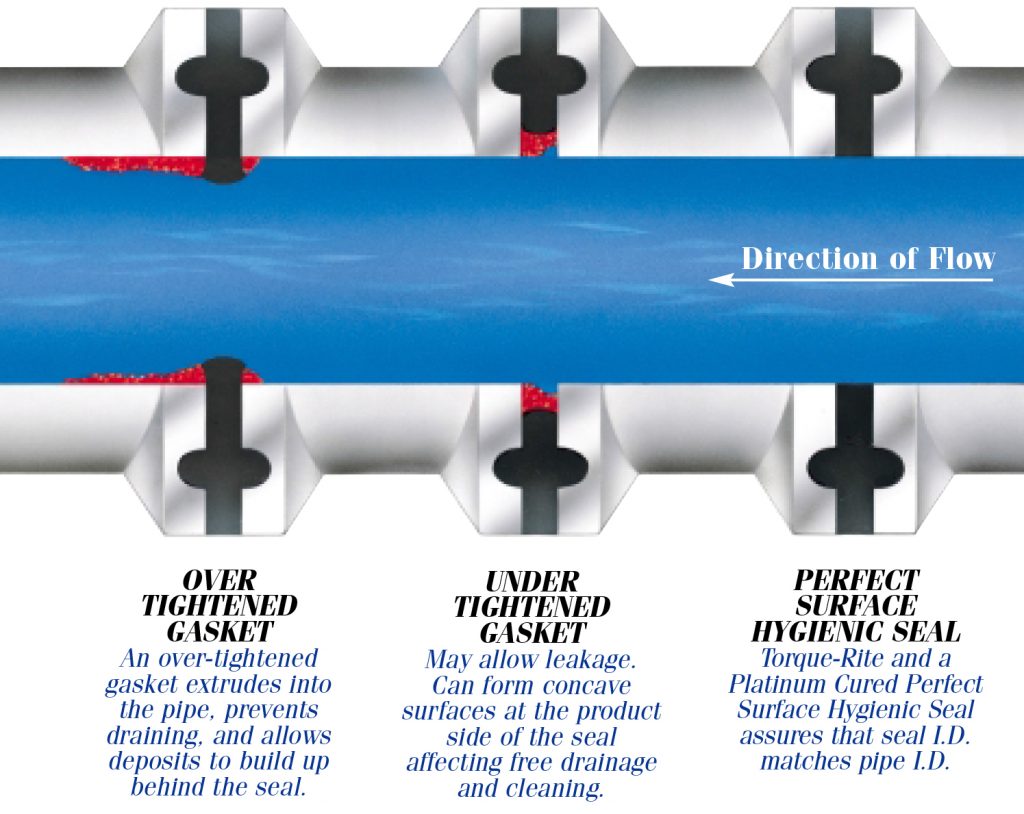
Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa
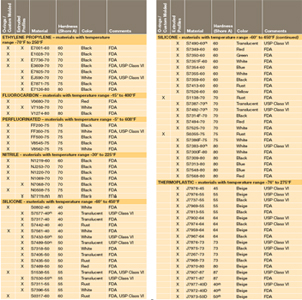
Fda Usda Nsf51 Usp Class Vi Compliant Seals Products
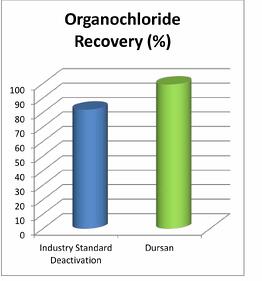
Dursan Passes Usp Class Vi Testing Why Is That Important

Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell